Introduction
In the last few decades, vascular surgery has made a dramatic shift from traditional open surgeries to percutaneous endovascular interventions that require internal access to an artery. Once the procedure is completed, the hole in the artery is closed by the traditional approach of applying manual pressure on the puncture site, or alternately, a Vascular Closure Device (VCD) is used to stop the bleeding. The traditional procedure is time consuming, painful and requires up to eight hours of bedrest, whereas VCDs stop bleeding quickly, thereby increasing patient comfort. However, complications such as rebleeding, hematoma formation, pseudoaneurysm, or retroperitoneal bleeding may arise during vascular closure procedures. It is mandatory for manufacturers and importers to submit to the USFDA, reports containing information on their marketed devices that cause serious injury or death on account of malfunctioning of their devices. All such reports are stored in a MAUDE (Manufacturer and User Facility Device Experience) database.
MAUDE
MAUDE is an online searchable database that is a repository of incidents caused by approved medical devices. It is mandatory for medical device manufacturers to submit safety and effectiveness data to the FDA, disclosing adverse effects on patients, and also submit a summary of the clinical studies. In addition to such reports that are submitted to the FDA during pre-market approval, manufacturers must also submit adverse events that happen during actual use of the device.
SciTech Patent Art (SPA) conducted a study to assess the safety and effectiveness of vascular closure devices (VCDs) by analyzing the adverse event data available in MAUDE database. While many studies on interpretation of MAUDE data are available in literature, they are based on the auto classification provided by database and are not accurate. In the current study, the adverse event reports were downloaded, read and reclassified manually. This approach has helped bring out better insights on device problems.
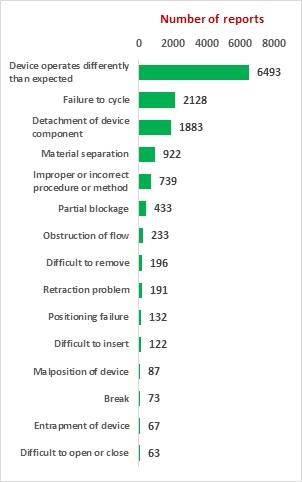
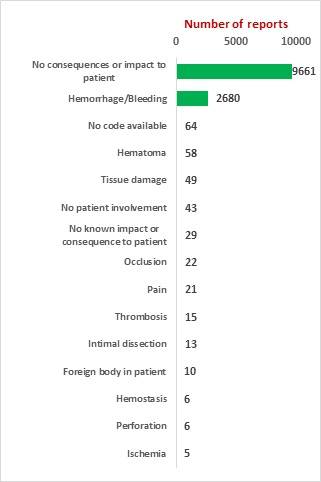
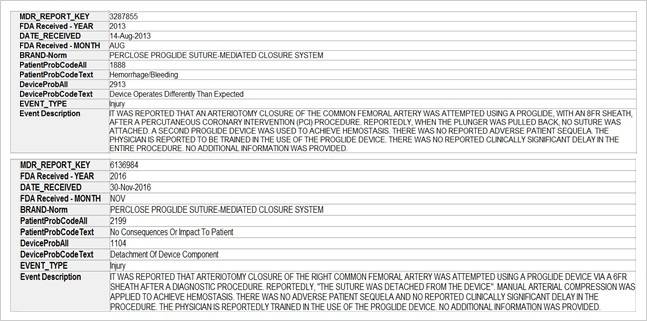
Study on Perclose ProGlide SMCS
The key market players in the vascular closure devices area are Terumo, Cardiva Medical, Abbott Laboratories, Cordis, Vivasure Medical, Teleflex, Vasorum Ltd and others. Abbott Laboratories has three different products, namely Perclose ProGlide Suture Mediated Closure (SMC) System, StarClose SE Vascular Closure System (VCS) and Prostar XL Percutaneous Vascular Surgical System (PVSS). Perclose ProGlide SMCS was selected for this study, as it is the most commonly used device for performing vascular closure procedures.