Abstract: Psychobiotics, a new class of probiotics, are gaining attention for their ability to influence the gut-brain connection and mental health, particularly for treating stress-related depression. Studies have shown that they can help balance mood and behavior by affecting gut bacteria and enhancing brain communication. They work by producing neurotransmitters, reducing neuroinflammation, and managing stress responses through the HPA axis. While results are encouraging, more research is needed to determine the best strains, doses, and treatment lengths. It is also important to assess their long-term safety and how they interact with traditional depression medications. Psychobiotics hold potential as a complementary or alternative option for managing stress-induced depression.
Introduction:
Depression, a common illness, technically called Major Depressive Disorder (MDD) has been affecting approximately 280 million people across the globe. Depression left untreated may escalate into a serious health condition, further leading to even suicide. Symptoms of depression include depressed moods (lost hope, sadness, and feeling of emptiness), low energy levels, lost enthusiasm or interest in activities, irregular sleep, changes in body weight and appetite. Depression can be mild, moderate, or severe depending on symptoms and individual’s personal experiences and functioning. [1] The available treatments of depression include therapies such as behavioral, interpersonal psychotherapy or visiting a professional or supervised face-to-face psychological treatment and drugs such as antidepressants. Serotonin reuptake inhibitors (SSRIs) and Tricyclic antidepressants (TCAs) are the commonly used antidepressants. In the case of most antidepressants, efficiency is not the same among all patients with mild to moderate symptoms and are often associated with several side effects.[2]
An alternative treatment for depression is emerging in the form of probiotics named Psychobiotics. Psychobiotics, when ingested, confer mental health benefit through interaction with commensal intestinal bacteria. Psychobiotics also include prebiotics that promote the growth of gut microbes. Research on Psychobiotics showed significant improvement of anxiety, depression, neurodegenerative and neurodevelopmental disorders, including Autism Spectrum Disorder (ASD), Parkinson’s Disease (PD) and Alzheimer’s Disease (AD). Recent study showed the role of probiotics in reversing aging of the brain.[3] Gut-brain signaling mechanism enables the psychobiotic effects. Lactobacillus brevis and Bifidobacterium dentium strains, the efficient GABA producers, are considered a promising therapeutic approach for depression.[4]

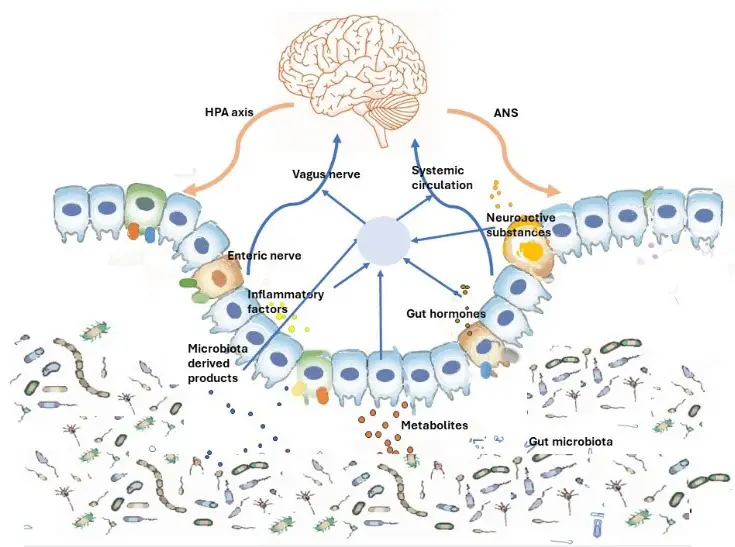
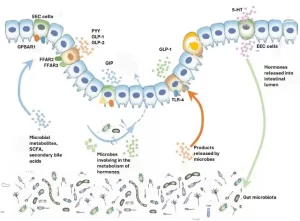
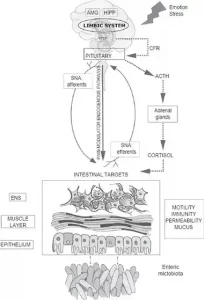 Gut-brain axis is influenced by the gut microbes which promote the release of biologically actives such as galanin and ghrelin from endocrine cells. Galanin stimulates the activity of HPA axis and thereby indirectly regulate the release of Adrenocorticotropic hormone (ACTH) and corticotropin-releasing factor (CRF) and also enhances glucocorticoid secretion from adrenal cortex.[14] Ghrelin contributes to the regulation of glucose homeostasis and metabolic stress.[15]
Gut-brain axis is influenced by the gut microbes which promote the release of biologically actives such as galanin and ghrelin from endocrine cells. Galanin stimulates the activity of HPA axis and thereby indirectly regulate the release of Adrenocorticotropic hormone (ACTH) and corticotropin-releasing factor (CRF) and also enhances glucocorticoid secretion from adrenal cortex.[14] Ghrelin contributes to the regulation of glucose homeostasis and metabolic stress.[15]

