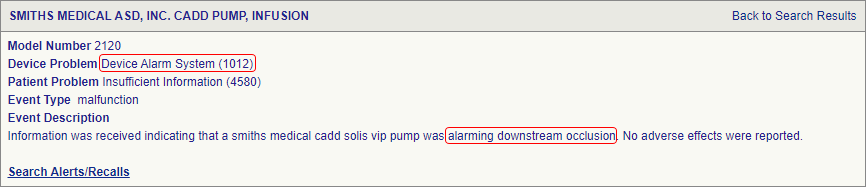
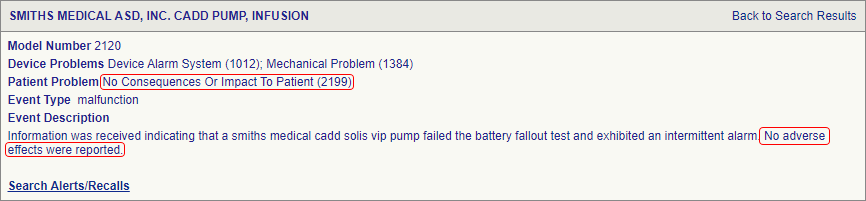
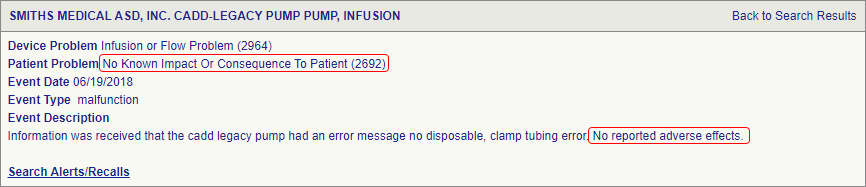
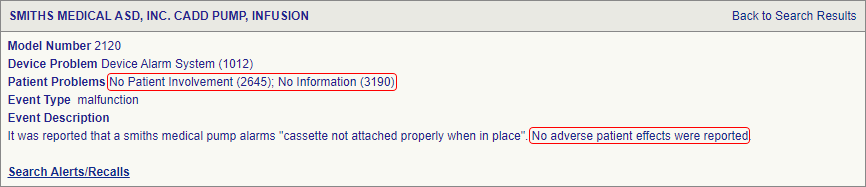
Introduction
Infusion pumps are used to treat diseases and disorders that require regular pharmacological intervention such as cancer, diabetes, and vascular, neurological, and metabolic disorders. Infusion pumps are frequently used to administer critical fluids including high-risk medications, and hence, pump failures can have significant implications on patient safety. Many infusion pumps are equipped with safety features such as alarms or other operator alerts that are intended to activate in the event of a problem. More recently, smart infusion pumps have been designed to alert the user when there is a risk of improper drug infusion or when the user sets the pump’s parameters outside of specified safety limits. These issues can compromise the safe use of infusion pumps and lead to over or under-infusion, missed treatments or delayed therapy. In the event of serious injury or death on account of malfunctioning of devices, it is mandatory for manufacturers and importers to submit to the USFDA, reports containing information on such events.
MAUDE
It is mandatory for medical device manufacturers to submit safety and effectiveness data to the FDA, disclosing adverse effects on patients during clinical trials, and submit a summary of the same. In addition to such reports that are submitted to the FDA during pre-market approval, manufacturers must also submit adverse events that occur during actual use of the device. These reports are available to the public on the MAUDE database which is a repository of incidents that were not experienced by approved medical devices during their premarket testing.
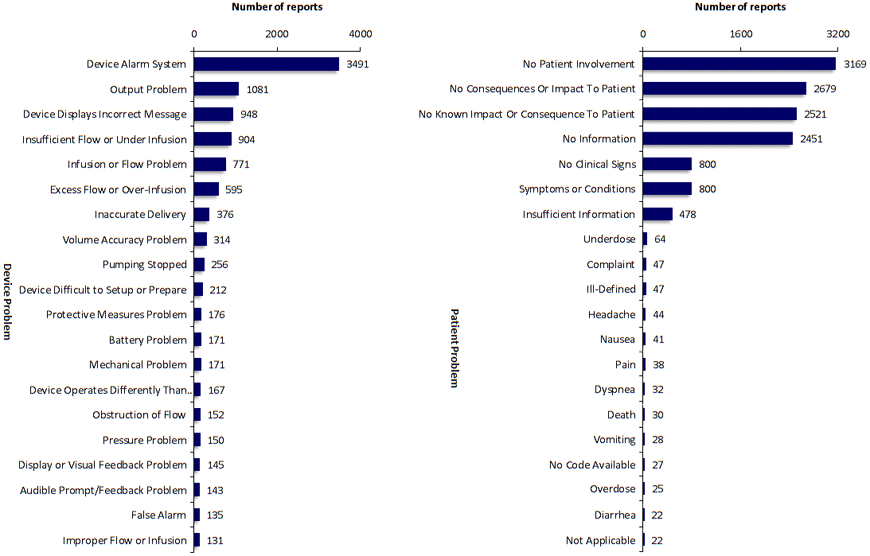
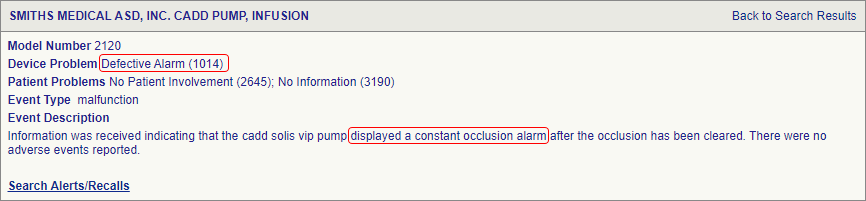
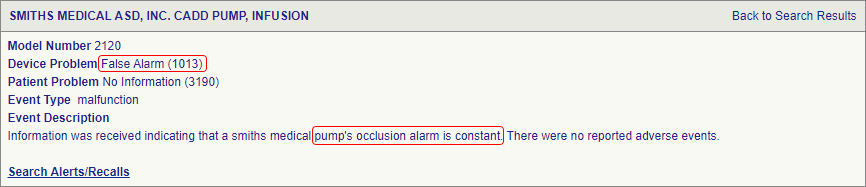
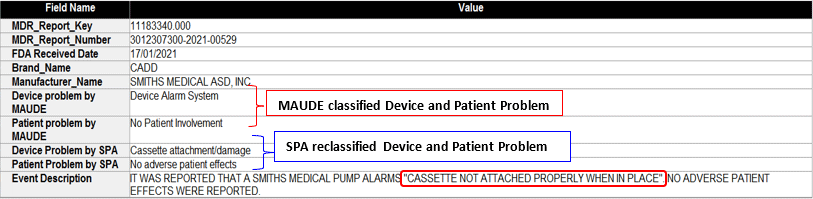
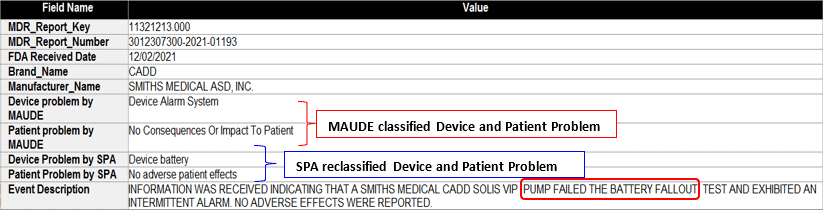
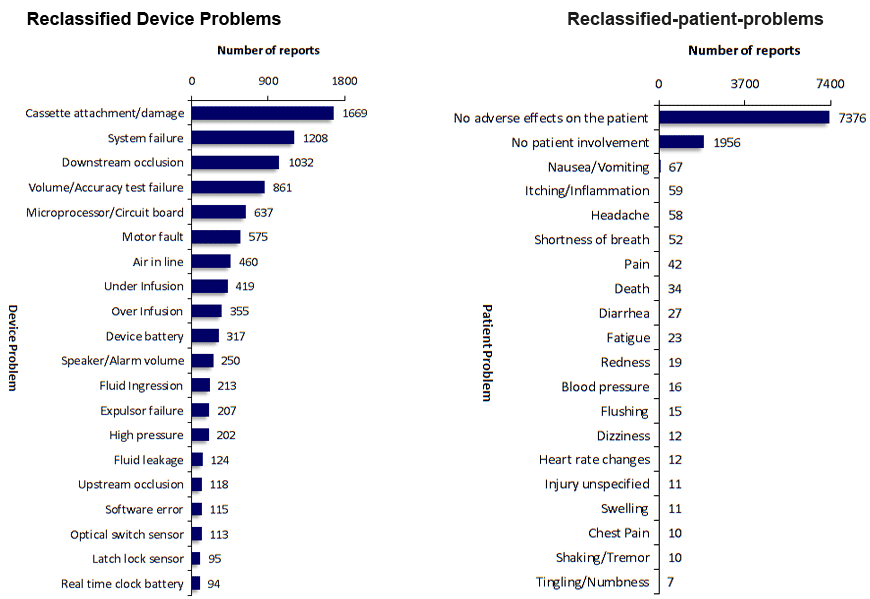
SciTech Patent Art (SPA) conducted a study to assess the safety and effectiveness of ambulatory infusion pumps of Smiths Medical, by analyzing the adverse event data available in the MAUDE database. While many studies based on the interpretation of MAUDE data are available in literature, they are not accurate because they are based on the auto classification provided by the database. To overcome this, SPA analysts downloaded the adverse event reports from MAUDE database, read the events and reclassified them manually. This approach helped them bring out better insights on device problems as compared to those based on automated classification.
Study on CADD® ambulatory infusion pumps
The key market players in the ambulatory infusion pumps area are Smiths Medical, Baxter, JMS, Becton, Dickinson and Company (BD), Tandem Diabetes Care, Medtronic, Roche, Bigfoot Biomedical, Animas Corp and others. Smith Medical manufactures CADD® ambulatory infusion pumps, Graseby™ syringe pumps, and Medfusion® infusion systems. Smiths Medical’s CADD® ambulatory infusion pumps were selected for this study, as they are the most widely used device for delivery of medication for cancer treatment, Parkinson’s disease, pain management therapies, etc. CADD® infusion pumps are used for intravenous, intra-arterial, subcutaneous, intraperitoneal administration, for sites that are in close proximity to nerves, into an intraoperative site (soft tissue, body cavity/surgical wound site), epidural or subarachnoid site. The pumps are intended for therapies that require a continuous rate of infusion, and/or an intermittent bolus, and/or with patient-controlled demand doses. Smiths Medical’s infusion pumps provide reliable performance and accurate medication delivery. They are compact, lightweight and easy to operate, so that the transition from hospital to home environment is easy (Fig.1).